
Blueprint Medtech Incubator Hub Cycle 4 Funding Solicitation
Posted Feb 1, 2024
The Center for Innovative NeuroTech Advancement (CINTA) & NeuroTech Harbor (NTH) announce the Cycle 4 Award Competition supported by the NIH Blueprint MedTech Program.
The Center for Innovative NeuroTech Advancement (CINTA) & NeuroTech Harbor (NTH) announce the Cycle 4 Award Competition supported by the NIH Blueprint MedTech Program. This program is seeking collaborative projects aimed at developing emerging technologies into commercially viable, clinically focused solutions for disorders of the nervous system that are aligned with the mission and interests of the participating NIH institutes and centers. [The next solicitation is expected to be announced Summer 2024.]
Overview
Innovators developing groundbreaking medical device technologies face several challenges along the translational path from bench to bedside. The NIH Blueprint MedTech: Incubator Hubs aim to address such challenges by accelerating the development of cutting-edge medical devices that will prevent, diagnose, and/or treat disorders involving the nervous system or consequences of such a disease or injury. This program will catalyze the translation of novel technologies from early-stage development to a prototype ready for first-in-human testing and will provide: (a) non-dilutive funds to support medical device development activities, (b) ongoing, specialized support from experienced executive mentors and (c) additional resources and support services including, but not limited to:
- Assistance from Hubs and consultants (e.g., on design, regulatory, reimbursement, intellectual property, commercialization, and strategic partnership issues).
- Resources to plan and support concept development, team building, needs assessment, and other early translational activities.
- Other resources listed on the Blueprint MedTech Website
The Center for Innovative NeuroTech Advancement (CINTA) and NeuroTech Harbor (NTH) are the two NIH-funded Incubator Hubs that will provide funding, mentoring, oversight, and in-kind resources to innovator teams. The objective of Hub support is to develop and de-risk these groundbreaking technologies to the point of first-in-human testing. Human subjects research beyond usability studies is unlikely to be supported through this funding. By the conclusion of funding, it is anticipated that projects will be ready to apply for funding for a companion solicitation from NIH (which support first-in-human evaluations of safety and effectiveness):
- Blueprint MedTech: Translator (UG3/UH3 – Clinical Trial Optional)
- Blueprint MedTech: Small Business Translator (U44 – Clinical Trial Optional)
or have secured or be ready to apply to other NIH or non-governmental funding sources.
Funding Opportunity
Two kinds of awards may be issued for applications submitted to this solicitation.
- Optimizer awards will rarely exceed $500,000 in direct costs per year for a period of up to 4 years. In addition to monetary support, awardees will receive mentoring and resources and support services necessary for translation will be available as outlined above.
- Seedling awards provide support for six months, a $25,000 stipend, and $25,000 to hire subject matter experts. Mentors will work with awardees throughout the project to help resolve specifically identified gap on the path to commercialization.
Principal Investigators (PIs) from academic institutions, industry, and non-profit organizations are invited to apply. Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Principal Investigator(s) is (are) invited to work with their organization to develop and submit a proposal.
The Blueprint MedTech Hubs and NIH encourage applications from women, underrepresented racial and ethnic groups, as well as individuals with disabilities.
Review Process and Eligibility
Applicants must submit pre-proposals, which will be used to consider eligibility. NIH program staff will determine whether the pre-proposal fits within the interests of the participating NIH institutes and centers and whether the pre-proposal is aligned with the scope of the BP MedTech program. Pre-proposals are submitted through a simple online application form equivalent to approximately three pages.
Eligible applicants will be invited to submit full proposals through the same online application system. The full proposal form is equivalent to approximately ten pages and will undergo review by CINTA and NTH. All full proposals will be considered for the Seedling program if the proof-of-concept data in the full proposal is sufficient, and reviewers identify any specific weaknesses in descriptions of the:
- Commercial opportunity
- Regulatory pathway
- Team
- Project Plan
Participants successfully completing the Seedling program may re-enter this program at the Full Application or Deep Dive stage or may be ready to apply to an NIH- Translator solicitation.
A subset of the applicants who submit full proposals will be selected to participate in an interactive “Deep Dive” evaluation, which is the final due diligence stage of review prior to funding decisions on Optimizer projects. Deep Dive evaluations will undergo review by CINTA and NTH.
Applicants should consider avenues to support diversity, equity, inclusion, and accessibility in their proposals. Applicants are encouraged to involve team members or collaborators from traditionally underrepresented groups in the translational workforce. Developing a product that considers population factors such as racial and ethnic groups, gender, and disabilities may further support diversity, equity, inclusion, and accessibility within the proposal. Additionally, proposals that address conditions or clinical indications where current preventative, diagnostic, or treatment options disproportionately fail to serve underrepresented populations are encouraged.
All reviews and discussions regarding pre-proposals, full proposals, and deep dives will be conducted on a confidential basis.
Timeline
Although the program is funded by NIH and NIH program staff play a role, the solicitation and evaluation process is quite unlike a traditional NIH R01 or R21 grant application. Full proposals will be assessed for the project’s commercial viability, including but not limited to the likelihood of technical de-risking and achieving a prototype ready for first-in-human testing, market need, regulatory pathway, potential clinical impact and for reducing treatment gaps, and projected costs to the payer. Reviewers are chosen based on their expertise in one or more of these areas and their comments and critiques focus on these aspects. Another difference from the NIH R01 or R21 grant process is that applications do not receive an overall impact score and only a brief review summary is provided.
Timeline
February 1, 2024: Pre-proposal submission opens
February 2024: Weekly informational webinars and Office hours by appointment
February 29, 2024: Pre-proposal Submission deadline
March 25, 2024: Submission opens for full proposals (by invitation only)
April 19, 2024: Full proposal Submission deadline
June-July 2024: Deep Dive Assessment
September 2024 (estimated): Final projects selected for funding
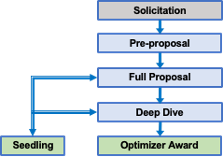
The above graphic represents the stages starting from pre-proposal to program selection.
Instructions
-
- Pre-proposals must be submitted through the Blueprint MedTech: Incubator Hubs Program online application system no later than 11:59 pm ET on February 29, 2024.The goal of projects should be a prototype ready for first-in-human studies.
- A subset of pre-proposals will be invited for full proposals, which are also submitted through the online proposal submission system.
- Selected full proposals will proceed to a “Deep Dive” review that is expected to take approximately three weeks. During this time applicants will need to commit significant effort to respond to the Deep Dive team’s inquiries.
- The earliest applicants will be informed of review decisions will be the end of October 2024.
- The earliest anticipated start date for funding for Seedlings is November 2024.
- The performance period for seedlings is 6 months.
- Seedling support will not exceed $50,000, split between funding to the proposers and consultants.
- The earliest anticipated start date for funding for Optimizer projects is February 2025.
- The anticipated performance period is up to 12 months, which can be renewed for up to an additional three 12-month periods with CINTA, NTH, and NIH approval.
- Optimizer awards will rarely exceed $500,000 per year in direct costs. Indirect costs will be provided at your institution’s Federally negotiated rate or, if none, a de minimis rate of 10%. We expect to make up to 10 awards from this solicitation.
Application Criteria
CINTA and NTH are seeking proposals leading to commercialization of groundbreaking preventative, therapeutic, and/or diagnostic medical devices. At a minimum, projects should have already clearly demonstrated proof-of-concept. The technologies proposed must have a pathway to a prototype ready for first-in-human testing within 4 years and have a viable pathway to commercial development. Projects that are nearly ready for first-in-human testing may be redirected to the companion NIH Blueprint MedTech: Translator FOAs or invited to the Seedling program to address specific weaknesses.
Applications must focus on an intended use involving the nervous system or addressing consequences of such a disease or injury in an area of interest of the Participating Institutes/Centers. Since applications outside the mission of these participating Institutes/Centers will not receive funding, applicants are encouraged to review the website. For questions about mission fit, contact the points of contact listed on the NIH Blueprint MedTech website prior to submitting a pre-proposal and indicate which specific disease is targeted by your product. All other questions should be submitted to info@blueprintneurotech.org.
Applicants must have collaborative teams incorporating product development and clinical expertise. Input from these collaborators must be clearly reflected throughout the applications and proposed scope of work. CINTA and NTH can assist in connecting potential applicants with potential collaborators if requested. Applicants from teams without clinical expertise are strongly encouraged to find clinical partners prior to submitting a pre-proposal.
Applications should:
- Address an area of interest of the NIH Participating Organizations.
- Identify the initial intended use (e.g., Beachhead Market) for the proposed device.
- Clearly define the current state of the art and its limitations, and highlight how the proposed technology will advance patient care.
- Include data showing that the proposed technology has already demonstrated proof of concept for the targeted indication with enough confidence that the remaining challenges are primarily related to preparations for first-in-human studies.
- Propose medical devices with first-of-its-kind technologies, novel intended use, or new safety aspects (typically De Novo, PMA, or possibly 510(k) requiring clinical data)
- Propose solutions that are equitable, affordable, and accessible to all clinically relevant communities, including underserved and vulnerable populations.
Applications will NOT be considered for:
- Products not regulated by the FDA.
- Fundamental basic/applied research projects prior to proof of concept.
- Device technologies that do not significantly advance the state of the art (e.g., device technology that proposes minor modifications to FDA-approved/cleared medical device technology).
- Animal model development: all in vivo animal models must be well established and characterized, and available to the applicant.
- Focused on technologies for functional augmentation of healthy individuals.
Please click here to sign up for upcoming webinars or to schedule time for office hours with program staff.
Please contact info@blueprintneurotech.org if you have any questions about the program.
