BluePrint MedTech FAQs
-
- Is this an NIH grant application? No. Solicitation, review, and oversight of awards is managed by the Blueprint MedTech incubator Hubs, CINTA and NeuroTech Harbor. Since funding for the Hubs is provided by NIH, the Hubs must comply with the NIH grants policy and flow these policies down to funded projects. These policies include financial management, protection of animal and human subjects, and other reporting requirements. If you are selected for funding, one of the two Hubs will sponsor your project and they will help you navigate these policies.
- How can I tell whether my product is mature enough for entry into Blueprint MedTech? We encourage solution concepts that have some degree of both clinical and technical proof of concept. Degree and nature of proof-of-concept evidence (clinical and technical) should be clear in the proposal. This program does not support basic or applied research (e.g., a typical NIH R01). Your proof-of-concept demonstration should rely upon established scientific or empirical results to the point where no further research is required, aside from a first-in-human demonstration. Please see the GAITS description of “proof of concept,” particularly the Technology “Demonstration Results” and “Key Component PoC Prototypes” descriptions for further guidance (www.gaits.org). We have developed this helpful graphic to help applicants determine which funding path is best suited for their needs.
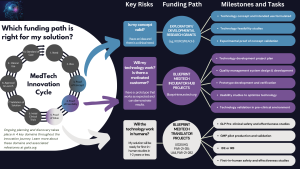
- How can I tell whether my product is too mature for entry into this incubator hub solicitation? If you are nearly ready for a clinical evaluation of safety and efficacy, we recommend submitting a grant application to PAR-21-282 and PAR-21-315. If the guidance in these NIH solicitations isn’t clear, please feel free to contact Blueprint-MedTech@nih.gov and describe what support you would need in order to begin that clinical study. In general, the pre-clinical activities supported by these NIH FOAs should involve few risks of failure (e.g., the design is frozen and you need to build prototypes and perform GLP animal studies)
- Who retains the rights to any intellectual property generated by a proposal? Management of intellectual property will be determined by the institutional policies of the proposing entity.
- Should I disclose any unprotected proprietary information in the submission? Protecting proprietary information is the responsibility of the applicant and the applicant institution. Consistent with NIH policy, applicants are discouraged from submitting information considered proprietary unless it is deemed essential for proper evaluation of the application. However, when the application contains information that constitutes trade secrets, or information that is commercial or financial, or information that is confidential or privileged, identify the pages in the application that contain this information by marking those paragraphs or lines with an asterisk (*) at the beginning of the paragraph. Indicate at the beginning of the Research Plan which pages contain asterisks and a note stating: “The following sections marked with an asterisk contain proprietary/privileged information that [name of applicant] requests not be released to persons outside the Government or the Blueprint MedTech incubator Hubs, except for purposes of review and evaluation.” When information in the application constitutes trade secrets or information that is commercial or financial, or information that is confidential or privileged, it is furnished to the Government and the Blueprint MedTech incubator Hubs in confidence with the understanding that the information shall be used or disclosed only for evaluation of this application. If a grant is awarded as a result of or in connection with the submission of this application, the Government shall have the right to use or disclose the information to the extent authorized by law.
- Are institutions/organizations/individuals outside of the United States eligible to participate? Foreign institutions, organizations, and individuals based outside of the United States may apply for this funding opportunity. Unfortunately, we can only reimburse up to eight percent of indirect expenses for foreign entities as per NIH grants policy.
- What happens if I miss the deadline for submitting a pre-proposal – can I still submit a full proposal? No. Pre-proposals are required; full proposals are invitation-only. We anticipate releasing the next Blueprint MedTech incubator hub solicitation in this winter.
- Do you have any accessibility accommodations? Please email info@blueprintneurotech.org for instructions if your input devices are having trouble with the submission system. No material can be submitted directly to Hub staff.
- Who reviews the full applications? The Review Panels are made up of practicing clinical specialists, engineers, medical device entrepreneurs, and scientists with broad experience in neurotechnology and medical innovation.
- Who should I list as collaborators? List only individuals who are considered key personnel or significant contributors to the work that will be performed, and who have already agreed to their role. Avoid honorific collaborations. For all proposals, the review committee requires that investigators collaborate closely with clinicians and product development experts in the development of the proposal and the conduct of the work. The input of these collaborators must be clearly reflected throughout the application.
- I am writing a pre-proposal, may I include several references? You can include as many references as you would like.
- Should I submit copies of relevant publications? Please do not submit copies of relevant publications. Standard citation of publications is acceptable.
- Are there any budget restrictions or limitations? Capital equipment must be well justified to the purpose and exclusive need of the project. Alterations and renovations will not be supported. Expenses for human subjects research will not be supported, aside from studies that meet the OHRP definition of “exempt.” See below for information about how other studies might be supported.
- Are indirect costs provided? Indirect costs will be provided at your institution’s Federally-negotiated rate. If your institution does not have a Federally-negotiated rate, indirect costs may be provided up to 10%. Foreign entities may only receive indirect costs up to 8%. Applicants may choose to waive their indirect costs.
- When would I receive my funding? Projects are estimated to be selected for funding no earlier than September of 2024. Your responsiveness to requests for administrative material will directly affect the timeliness of funding. If your proposed work involves human subjects research or vertebrate animal research (including data or tissue derived from humans or animals), the necessary IRB and IACUC approvals must be received prior to award.
- What are my reporting responsibilities if I receive an award? An annual report will be required in accordance with the terms and conditions of the award. Quarterly reports will be required to facilitate progress and integration with the overall program. Project teams are expected to attend an annual meeting hosted in either Boston or the Baltimore/Washington region. Please include reporting and annual meeting expenses in your full proposal’s budget.
- Are there any study restrictions or limitations? Human subjects research will not be supported, with the exception of IRB-exempt or minimal-risk clinical studies (as defined by IRBs). Note that minimal-risk clinical studies must be conducted at Georgia Tech HomeLab, one of the core resources of the Blueprint Medtech program. Do not include the cost of HomeLab studies in your budget. All animal models proposed for funding should already be well-validated. We will not support the development of new animal models.
- Are there any considerations specific to projects addressing mental health disorders? If your project addresses a mental health disorder, you are encouraged to use quantitative neural functional measures (e.g.., fMRI), and cross-diagnostic measures that have been well-validated with clinical outcomes, i.e., a trait that occurs across different psychiatric diagnoses.
- What are the DEIA objectives of this program? A major emphasis of this program is to create opportunities for diverse innovators, including those who have been traditionally underrepresented in the neurotech space and address conditions or clinical indications where current diagnostic or treatment options disproportionately fail to serve underrepresented populations. Applicant teams are encouraged to examine the makeup and diversity of their team, and work to seek out partners to increase the diversity of their team. Additionally, solution descriptions should include how the solution will be accessible and acceptable to underserved communities or describe a plan to make it so.
- What is a PEDP? A “Plan for Enhancing Diverse Perspectives” (PEDP) is a summary of strategies to advance the scientific and technical merit of the proposed project through inclusivity. All full proposals are required to provide a one-page PEDP. Key elements and examples can be found on the BRAIN Initiative’s website.
- Will you accept a video showing how my system works? Videos, images and additional information may be uploaded (file limitations apply).
- What is an institutional sign-off page? It is the official sheet from your research administration office indicating that your full proposal is approved by your institution. Be sure to ask your research administration office how much lead time they need to process this form in the event you are invited to submit a full proposal so that you can submit all your documents before the deadline. Signature from an authorized business official must be received prior to the proposal deadline. Institutional sign-off for pre-proposals is not needed.
For small businesses that do not have an institutional sign-off process, endorsement by signature from a member of the company’s leadership is acceptable. - Do I need to specify what medical condition would be diagnosed and/or treated by my product? Yes. We encourage you to have a clear disease or set of diseases that would be addressed by your product, and to state that explicitly. While you may have plans to eventually expand into other indications, it is best to stay focused on your initial product launch.
- Who do I contact if my question isn’t addressed in the FAQ? Please contact info@blueprintneurotech.org. As this solicitation is from the Blueprint MedTech incubator hubs, you should only contact NIH personnel to determine if your product’s intended use is supported by a specific Institute or Center.
- Are there any budget restrictions or limitations? Capital equipment must be well justified to the purpose and exclusive need of the project. Building alterations and renovations will not be supported. Expenses for human subjects research will not be supported, aside from studies that meet the OHRP definition of “exempt.” See below for information about how other studies might be supported.
Full Proposal FAQs
- The budget has a character limit of up to 500 words and no specific budget template – is there guidance on how it should be created? Budgets can be presented in a variety of ways, but should aim to describe costs based on milestones summarized per year. If character limits allow, a further breakdown of costs can be developed by grouping into cost categories such as: personnel/effort, fringe, materials/supplies, travel, indirect costs, etc.
